 enEnglish
enEnglish
JRS Pharma Media Library
For more information about the product, you may download the brochure, Technical Information, Formulation Handbooks or watch den videos and the SEM pictures about our products. If you need more information, please contact us.



Acyclovir Suspension
Acyclovir is an antiviral drug that is active against the herpes virus.

Adjustment of Rheological Properties for Veterinary Suspensions
Oral suspensions, or drenches, are a popular and common approach to overcoming the many obstacles of administering medicines to animals.





A Rational Approach to Excipient Selection
When developing a formulation for an immediate-release tablet, critical attributes of the active ingredient, including concentration, compactability, flowability, and solubility typically gu



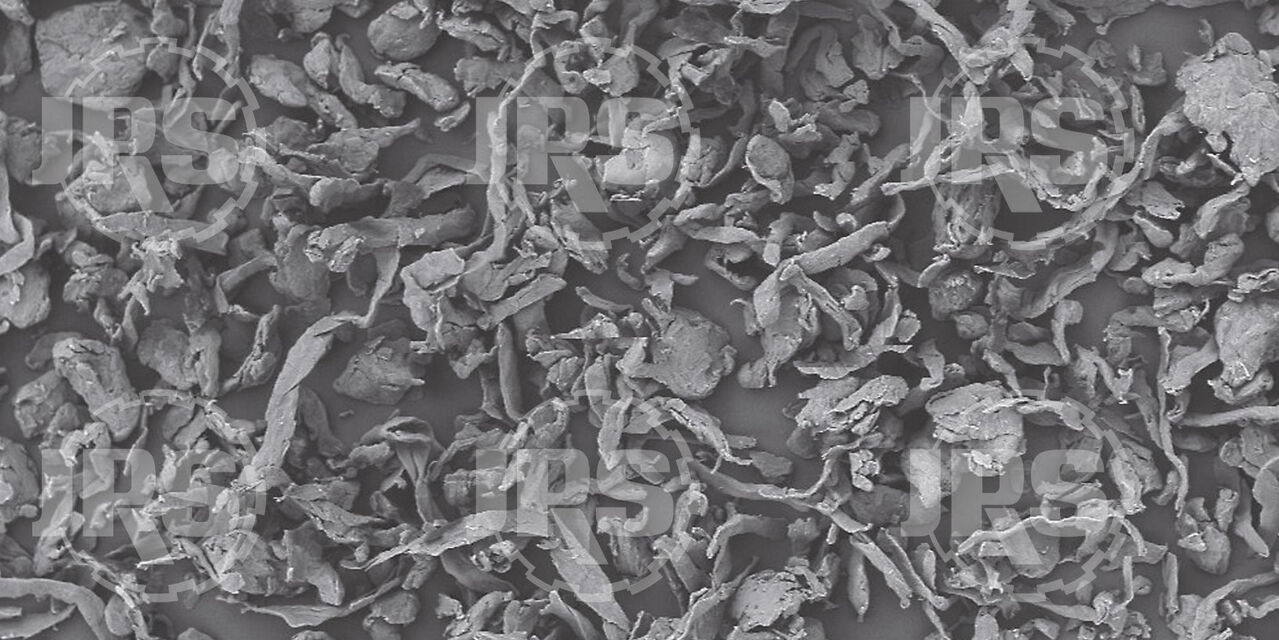



Best Practices for Effective Tablet Lubrications
Lubricants play a vital role in drug tableting applications; however, they can also produce disastrous results if not fully understood and used carefully.

Blending Efficacy of PROSOLV® SMCC with Caffeine by Near-Infrared Spec
PROSOLV® SMCC produced blends with better uniformity (as measured by NIRS) than the EMCOCEL® -CSD blends.



Cellulose in Tableting Technology
The development of co-processed, multifunctional excipients has enabled formulators to address multiple challenges with a single excipient, resulting in enhanced production and better finish




